Supported Liquid Extraction
Sample preparation using Supported Liquid Extraction
The Supported Liquid Extraction (SLE) process is analogous to traditional liquid-liquid extraction (LLE) and utilizes the same water immiscible solvent systems for analyte extraction. However, instead of shaking the two immiscible phases together, the aqueous phase is immobilized on an inert diatomaceous earth based support material and the water immiscible organic phase flows through the support, alleviating many of the liquid handling issues associated with traditional LLE such as emulsion formation. As a result recoveries are often higher and demonstrate better reproducibility from sample to sample.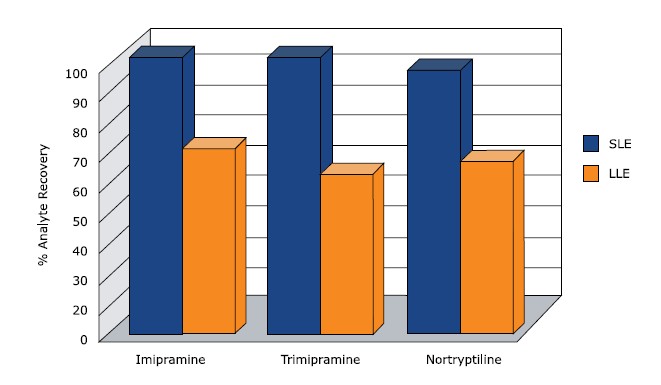
Recovery of tricyclic antidepressants from plasma, typical ISOLUTE SLE+ procedure compared with the equivalent LLE procedure
In sample preparation, the principles of traditional LLE (partitioning of analytes between aqueous and water immiscible organic solvents) are well known and understood. Traditionally, analytes are extracted from aqueous samples through the addition of an appropriate water immiscible organic solvent. The two immiscible phases are shaken or mixed thoroughly in a separating funnel, and based on relative solubility of the analytes in the two phases, analytes will partition into the organic solvent. The efficiency of the extraction is enhanced by the shaking, which creates a high surface area for the extraction interface allowing partitioning to occur. LLE can give particularly clean extracts of biological fluids, since matrix components such as proteins and phospholipids are not soluble in typical LLE solvents, and are therefore excluded from the final extract. The same benefits are true for supported liquid extraction (SLE) procedures: because the same water immiscible solvents are used in SLE, proteins and phospholipids are efficiently removed from the final extract, and no additional steps such as protein crash (precipitation) are required.
Using a fast, simple Load-Wait-Elute procedure, Supported Liquid extraction using ISOLUTE SLE+ products provides inherently cleaner extracts than other simple sample preparation techniques such as ‘dilute and shoot’ or protein precipitation.
The efficient extraction process combining high analyte recoveries, elimination of emulsion formation, and complete removal of matrix interferences such as proteins, phospholipids, and salts results in lower limits of quantitation compared to traditional LLE.
ISOLUTE SLE+ products contain a modified form of diatomaceous earth, which provides a support for the liquid liquid extraction process to occur, but does not interact chemically with the aqueous sample.
Application of the sample to the column results in the aqueous sample spreading over the surface of the material, forming an immobilized layer of small droplets held in place by a network of pores.
Supported Liquid Extraction mechanism of action
When the water immiscible extraction solvent is applied for the elution step, it flows over the aqueous droplets allowing efficient analyte partitioning. The large surface area of the extraction interface and flow through nature of the technique leads to a very efficient extraction procedure, because analytes come into contact with fresh solvent as the organic phase travels through the bed, mimicking a repeat LLE mechanism.
Excellent removal of phospholipids and proteins
Although SLE is a very simple sample preparation technique, it provides much cleaner extracts than techniques such as ‘dilute and shoot’ and protein precipitation. Extract cleanliness can often be comparable with more selective SPE approaches. In SLE, analytes are extracted by partitioning into a water immiscible organic solvent. Endogenous components such as salts, proteins peptides and phospholipids (present in blood related matrices) which cause matrix effects in LC-MS/MS are not soluble in these solvents. As a result they remain in the aqueous phase on the sorbent and are therefore eliminated from the final extract. High analyte recoveries along with low matrix components and reduced ion suppression result in better quantitation and method performance.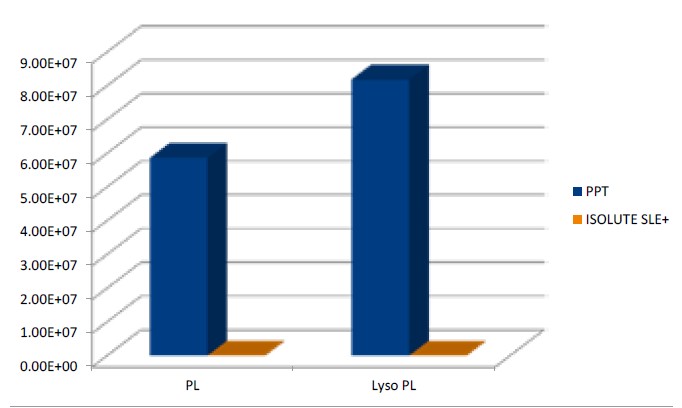
Comparison of phospholipids and lysophospholipids present in sample extracts prepared using protein precipitation (PPT, 100μL human plasma precipitated with 300μL acetonitrile) and ISOLUTE SLE+ (100 μL human plasma extracted using MTBE)
To demonstrate the effectiveness of ISOLUTE SLE+ in removing proteins from biological fluid samples, serum was prepared using ISOLUTE SLE+, protein precipitation and solid phase extraction. Residual protein in the extracts was analyzed using gel electrophoresis. Complete protein removal was seen with ISOLUTE SLE+.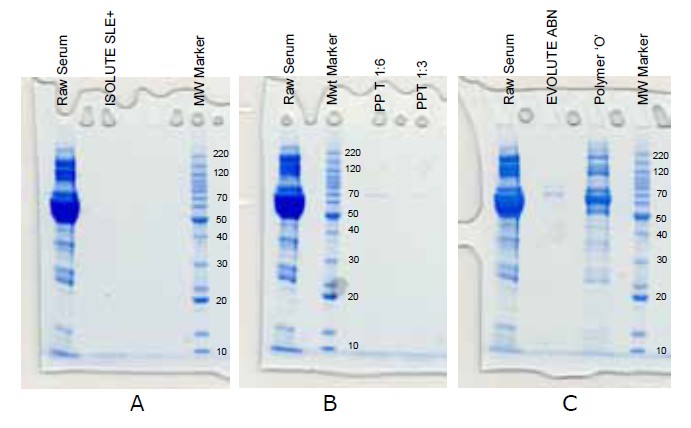
Protein removal, ISOLUTE SLE+ vs. protein precipitation and solid phase extraction.
(A) ISOLUTE® SLE+: Rat serum pre-treated 1:1 (v/v) with water, loaded onto ISOLUTE SLE+ plate and extracted with MTBE. All extracts were evaporated to dryness for gel electrophoresis work up.
(B) Protein precipitation: rat serum precipitated with acetonitrile (1:3 and 1:6 v/v)
(C) Solid phase extraction: 100 μL rat serum extracted using manufacturers recommended generic methods
Gel electrophoresis: NuPAGE Novex 12% Bis-Tris mini gel with MOPS SDS running buffer at 200V, 120mA and 12.5V. Gels were run for approximately 65 minutes to ensure complete protein migration.
